For most people, aging means inevitable and irreversible decline. Our bodies wear down, our cells malfunction and we become more susceptible to diseases. But new research from the emerging field of geroscience is modifying this narrative. If this new research bears fruit, it would mean progress toward a goal that’s as old as humanity: to add years to people’s lives, and ensure that those years are filled with vitality and independence.
Geroscience rests on the notion that aging itself, rather than individual diseases, is the root cause of many chronic conditions. To explore this hypothesis, scientists have focused on understanding and targeting the biological mechanisms of aging with the goal of extending both lifespan and healthspan, or the number of healthy years.
Extending healthspan would improve the quality of life for millions of people and reduce the burden of aging-related diseases. Baby boomers, most now well beyond the age of 65, are grappling with conditions like heart disease, type 2 diabetes, cancer, Alzheimer’s and frailty, which not only reduce quality of life but place immense pressure on families, healthcare systems and economies.
Many geroscientists believe that the biological mechanisms of aging are both malleable and treatable. Researchers in clinics and laboratories around the world are now testing this notion. If it turns out to be correct, it could be possible to slow aging or even reverse it.
Targeting aging itself
Since biological aging is the primary mechanism for the emergence of chronic diseases over time, according to the geroscience hypothesis, understanding its associated molecular pathways is essential. By addressing these underlying processes, we believe we may be able to slow aging itself and delay the onset of heart disease, diabetes, Alzheimer’s and many other aging-related diseases. Rather than tackling each one of them individually, we should make what’s common to them all the main target: the aging process.
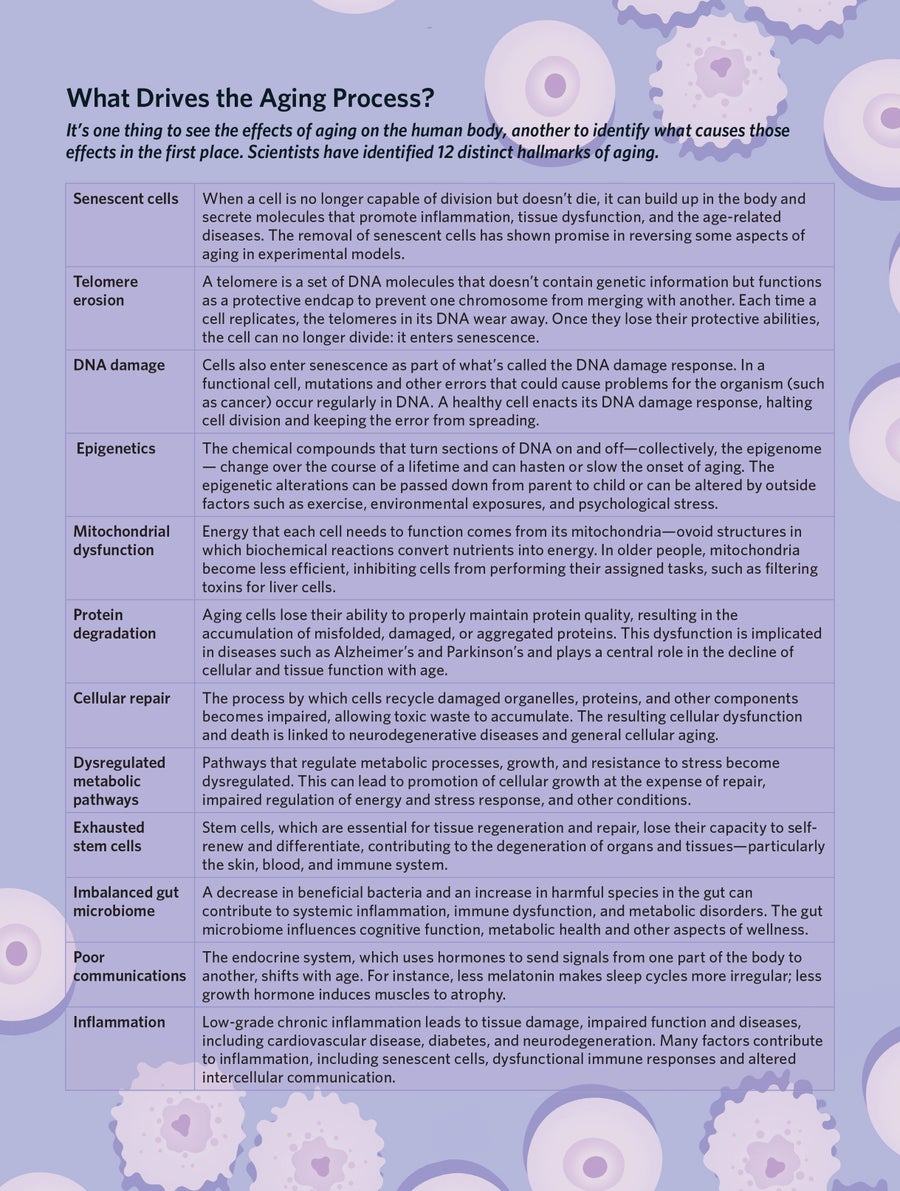
Research has shown that aging is driven by a series of interconnected biological processes that begin early in life and build up over time. Scientists have identified a dozen of these processes, often referred to as the hallmarks of aging. As our bodies age, damage accumulates to our DNA from radiation, errors during replication and oxidative stress. Our mechanisms of repairing this damage grow less efficient. Protective caps on the ends of our chromosomes, called telomeres, shorten. The ability of our cells to produce proteins to carry out metabolic functions degrades. Our mitochondria—the powerhouse of our cells—can become dysfunctional. The stem cells necessary for tissue regeneration and repair lose their capacity to self-renew and differentiate. Our gut bacteria can become imbalanced. The ability of our cells to communicate with one another can be disrupted.
These developments increase our bodies’ susceptibility to disease. Many of them promote inflammation, inhibit tissue repair, promote insulin resistance and impair our stress response. These changes can increase our susceptibility to a host of age-related diseases, including neurodegenerative disease like Parkinson’s and Alzheimer’s, as well as diabetes, cardiovascular disease, macular degeneration and many forms of cancer.
By deepening our knowledge of these processes of aging, we are identifying targets for treatments that slow them down. One promising target is senescent cells. These are cells that stop dividing but refuse to die. Instead, they remain active, secreting molecules that lead to inflammation and contributing to tissue damage and age-related diseases. Scientists believe that the accumulation of these senescent cells is a major driver of aging and its associated disorders.
The new field of senolytics involves designing drugs that can clear senescent cells out of the body. The late biochemist Judith Campisi and her colleagues at the Buck Institute have successfully used these strategies to extend the lifespan and healthspan of laboratory animals. Unity Biotechnology, a startup, has also successfully targeted an age-related eye disease with senolytics in phase 2 clinical trials (the authors have no relationship to Unity). If all goes well, the work could lead in a few years to treatments to clear out these harmful senescent cells.
Calorie restriction (CR) is one of the most robust interventions for extending lifespan and delaying age-related diseases across a variety of organisms, from yeast to primates. By reducing caloric intake by 20 to 40 percent without malnutrition, CR triggers profound physiological changes, including enhanced autophagy, improved mitochondrial function and reduced oxidative stress. These effects slow cellular damage and improve metabolic efficiency, contributing to delayed onset of conditions such as cardiovascular disease, diabetes, cancer and neurodegenerative disorders. Studies in humans, such as the CALERIE trials, suggest CR can improve markers of aging, like reduced inflammation and improved insulin sensitivity, though long-term adherence remains a challenge.
Emerging therapies like metformin and rapamycin seek to replicate CR’s benefits without the need for strict dietary intervention. Metformin, a widely used anti-diabetic drug, activates AMPK, a cellular energy sensor, while dampening mTOR signaling—key pathways implicated in aging. By improving glucose metabolism, reducing oxidative stress and modulating gene expression, metformin shows promise in delaying age-related diseases. The TAME trial is currently testing its potential to reduce the risk of conditions such as cardiovascular disease and cognitive decline in humans.
Rapamycin, a potent inhibitor of mTOR, also mimics CR’s effects and extends lifespan in multiple species, even when administered late in life. It enhances autophagy, reduces cellular senescence and improves immune function. Clinical studies have shown rapamycin can rejuvenate the immune system in older adults and may mitigate age-related cardiac dysfunction. However, its immunosuppressive effects require careful monitoring.
Together, CR, metformin and rapamycin target the fundamental mechanisms of aging, offering hope for interventions that could extend healthspan and reduce the burden of age-associated diseases.
Tailored interventions
Aging is not a one-size-fits-all process; genetic factors and lifestyle choices also play a role in how fast or slowly we age. Once we have identified specific factors that influence an individual’s aging process, it may be possible to tailor interventions that target the unique biological mechanisms at play in each person. Since the aging process happens slowly, a preventative approach may be able to head off the onset of aging-related diseases and extend the healthy years of life. In addition to aging, other risk factors for disease are specific to the individual, including genetic background and lifestyle. This personalized approach to aging could lead to a new era of medicine, where treatments are designed to extend life and improve its quality by reducing the burden of age-related diseases.
Significant challenges remain. Limited access to treatments could worsen social inequalities. Since age is not a disease per se, current regulations do not currently accommodate treatments for it. For geroscience to truly take hold, regulators will need to adapt to this new way of thinking about health.
By targeting the fundamental processes that drive aging, we have the potential to transform medicine and society. The future could see longer, healthier and more vibrant lives, where aging is no longer associated with decline but with continued growth and fulfillment.
Text by Eric Verdin and Gordon Lithgow; Illustration by Joey Guidone
Learn more about the Buck Institute's pioneering research on aging and longevity here.
Explore the emerging science of healthspan in other stories in this special report.



