ABSTRACT
The development of the gut microbiome is crucial to human health, particularly during the first three years of life. Given its role in immune development, disturbances in the establishment process of the gut microbiome may have long term consequences. This review summarizes evidence for these claims, highlighting compositional changes of the gut microbiome during this critical period of life as well as factors that affect gut microbiome development. Based on human and animal data, we conclude that the early-life microbiome is a determinant of long-term health, impacting physiological, metabolic, and immune processes. The early-life gut microbiome field faces challenges. Some of these challenges are technical, such as lack of standardized stool collection protocols, inconsistent DNA extraction methods, and outdated sequencing technologies. Other challenges are methodological: small sample sizes, lack of longitudinal studies, and poor control of confounding variables. To address these limitations, we advocate for more robust research methodologies to better understand the microbiome’s role in health and disease. Improved methods will lead to more reliable microbiome studies and a deeper understanding of its impact on health outcomes.
KEYWORDS: Infant microbiome, immune development, gut microbiota, chronic conditions, early life gut microbiome, cesarean delivery microbiome, microbiome diversity, childhood allergies, probiotics and prebiotics interventions
Introduction
The human gut microbiome is a complex and dynamic ecosystem that begins to take shape during birth and undergoes significant developments during the first three years of life1–3; .4 After birth, a relatively defined pattern of microbial succession can be observed, particularly in breastfed infants. Hallmarks for these are initial colonization in the first weeks with facultative anaerobes followed by generally a dominance of Bifidobacterium in the first 6 months of life, again followed by the establishment of a diverse community of species from the main human gut phyla, Bacteroidota and Firmicutes, as solid foods are introduced. When established properly, the gut microbiome contributes to the digestion of dietary fiber and other carbohydrates that are indigestible by host enzymes. It also plays a key role in nutrient absorption, immune system development, metabolism regulation, and pathogen colonization prevention.5–9 However, many of the factors that play a role in this establishment, development, and resilience are not well understood. What is clear is that the mode of delivery (vaginal or cesarean),10,11 feeding practices (breast milk or formula),7,12,13 gestational age,14–16 maternal microbiome,17,18 maternal diet,19–21 antibiotic exposure,12,22 and lifestyle23–26 are key determinants. Consequently, perturbations of microbiome establishment are associated with increased risks of chronic conditions, for example asthma,27,28 atopy,29,30 diabetes,31 and obesity.32,33 Because of the potential to prevent such chronic diseases, there is broad interest in interventions to positively influence the microbiome’s early development, including education around breastfeeding, dietary and lifestyle changes, and suggestions to use prebiotics and/or specific strains of probiotics.34–38
The premise of this review is that the early-life gut microbiome not only supports initial growth and immune development but also sets the stage for long-term health outcomes. This premise is supported by a large body of literature, from associative studies in humans to mechanistic work in animal models. To review this and provide a vision for future science and innovation in this space, we first highlight key studies describing the composition and functions of the gut microbiome during the first three years of life, factors influencing the microbiome, and the health outcomes associated with its disturbed development. We subsequently discuss strategies for microbiome intervention, identify shortcomings of current studies and suggest improvements to this, and examine the question of whether there exists an ideal early-life gut microbiome. Overall we believe optimizing the early life gut microbiome will have profound positive consequences for future health.
Composition of the early life gut microbiome
This section covers the dynamics and succession patterns of initial gut microbiome development, starting from the neonatal period, through early and mid-infancy, and finally into toddlerhood. Shaped by factors such as delivery mode, feeding methods, and environmental influences, the gut microbiome undergoes significant changes during the first three years of life (Figure 1).
Figure 1.
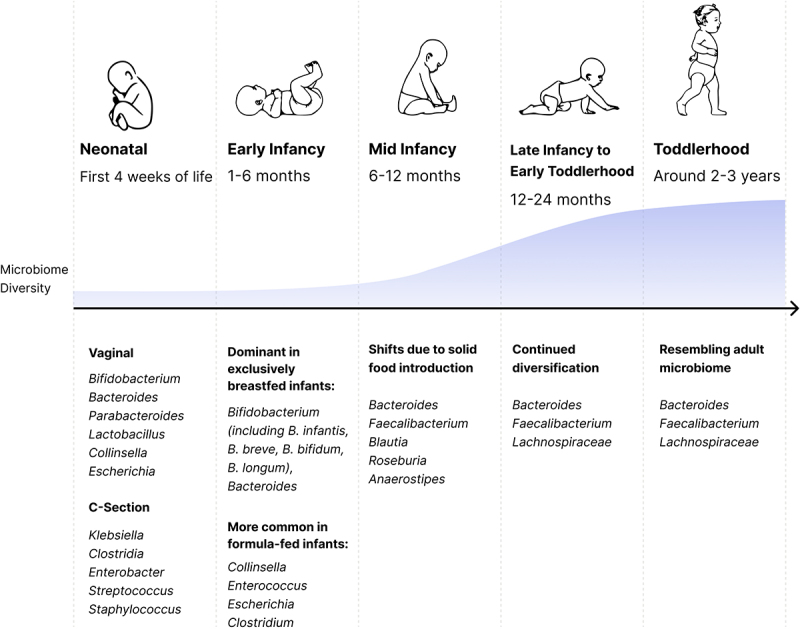
Microbiome maturation in the first 1000 days. Listed taxa names are the most abundant at the population level but drastic variation between individuals exists.
In the first 4 weeks of life, the gut microbiome’s composition varies widely based on delivery mode. In vaginally-born infants the gut microbiome is composed of a stochastic mixture of microorganisms principally transmitted from the mother’s gut and other secondary sources, like the oral cavity and vagina.11,39,40 As such, members of the genus Bifidobacterium, Bacteroides, Lactobacillus, Clostridium, and Escherichia are often observed.2,4,41–43 In contrast, infants born via Cesarean section (C-section) tend to contain more species of Klebsiella, Clostridium, Enterobacter, Staphylococcus and other opportunistic pathogens in their gut microbiome, potentially originating from the skin, oral canal, and the hospital environment.4,11,41,44 Importantly, trends described here are group averages and obscure the fact that the gut microbiome of many infants is widely diverging from the mean, and infant microbiomes have even larger variation in Unifrac and Bray-Curtis beta diversity metrics than what is observed for adult’s microbiomes.13,45–47 Over time the microbiome of Cesarean-born infants tends to become similar to those born vaginally, showing increasing similarity at 6 months and one year, even though differences in specific signatures and species have been shown to persist up to 2 years in a subset of children.11,48,49
During early infancy (1–6 months), the infant’s microbiome profile shifts. More strict anaerobes, such as species from genera Bifidobacterium, Bacteroides, and Clostridium, become more dominant; while facultative anaerobic microorganisms, like species of the genus Escherichia, decline.50 This stage is highly influenced by the feeding method. The gut microbiome of exclusively breastfed infants is dominated by members of the genera Bifidobacterium and Bacteroides.7,12,13,47,51–53 Among these, Bifidobacterium species that have the capacity to degrade human milk oligosaccharides (HMOs—B. breve, B. longum subspecies infantis, B. bifidum, and B. longum—make up the majority.7,13 In contrast, formula-fed babies have lower levels of Bifidobacterium and Bacteroides, a higher Shannon diversity, and are more frequently colonized by members of the genera Collinsella, Enterococcus, Escherichia, and Clostridium.7,13,51,52
Mid infancy, typically from 6 to 12 months, is marked by the introduction of solid foods, leading to an increase in microbiome diversity and shifts in taxa from breast milk degraders to a more diverse community. This includes increased abundance of members from genera Bacteroides, Faecalibacterium, Blautia, Roseburia, Anaerostipes, and other anaerobes capable of metabolizing complex starches and fibers present in solid foods.7,13,46 While weaning is a big driver of microbiome diversification,54,55 this transition occurs in both breastfed babies and formula-fed infants, since novel solid foods create new nutrient niches for microbial colonization.
During late infancy and early toddlerhood, from 12 to 24 months, the gut microbiome continues to diversify.7,42,46,56 This is driven by dietary changes as the child’s diet becomes more varied. By around 2 to 3 years, the gut microbiome stabilizes and, for some children, becomes indistinguishable from that of an adult,7,57 characterized by a predominance of the genera Bacteroides and Faecalibacterium, and members of the Lachnospiraceae family.7,42,56
However, observations based on taxa can obscure significant functional variability. For example, Bifidobacterium species and strains differ in their abilities to metabolize HMOs and produce SCFAs. Unraveling this functional variability requires refined analyses, for example leveraging metagenomics and metatranscriptomics. In the following section, we will explore the specific functions provided by the early life gut microbiome, exploring its core functional contributions to the host.
Which functions are provided by a healthy early life gut microbiome?
The early life gut microbiome performs key functions during a critical developmental period. These functions include the breakdown of complex carbohydrates, facilitation of nutrient absorption, inducing colonization resistance, and modulation of the immune system. In this section we will review these functions in detail.
HMO degradation
Breast milk contains a diverse array of nutrients and bioactive compounds contributing to infant development.58 Among its components are HMOs, complex carbohydrates that constitute the third most abundant component of breast milk, after lactose and fats.58 More than 200 different HMOs have been identified,59 with structural variations in their core structure, length, size, and glycosidic linkages.60 HMOs are indigestible by the human digestive system and thus serve directly to shape the developing gut microbiome. HMOs exert direct effects on the host or act indirectly by affecting bacteria species or functions.61
HMOs act directly through their antimicrobial properties against pathogens, inhibiting pathogen colonization by functioning as a soluble decoy receptor. Experiments conducted in vitro have shown that they stimulate dendritic cell function, promote the production of interleukins (e.g., IL-10, IL-27, and IL-6), and can modulate toll-like receptor (TLR) expression; influencing immune recognition and response to pathogens.62 For instance Sodhi et al. showed that HMOs 2’-fucosyllactose (2’−FL) and 6’-sialyllactose (6’−SL) reduce inflammation by inhibiting TLR4 signaling in vivo.63
Beyond their direct role, HMOs exert indirect effects through the metabolites produced by their fermentation by the gut microbiome, specifically short-chain fatty acids (SCFAs), and the cross-feeding interactions these metabolites facilitate within the gut microbiota. HMOs undergo saccharolytic fermentation by gut microbes, resulting in the production of SCFAs such as acetate, butyrate, and propionate; whose production varies depending on the infant’s age.64 These SCFAs serve as energy sources for colonocytes, promote anti-inflammatory cytokine production, and inhibit pathogen growth.65 In the healthy early-life gut microbiome, specific bacterial taxa such as Bifidobacterium and Bacteroides species play a key role in HMO breakdown. These bacteria possess a diverse array of genes encoding glycoside hydrolase (GH) enzymes, collectively enabling the degradation of a wide spectrum of HMO structures.66 The location of GHs can vary by species, being either intracellular or cell-wall anchored. Intracellular GHs hydrolyze intact HMOs that are transported into the cell into monosaccharides that can be used for energy metabolism. In contrast, cell-wall anchored GHs break down HMOs extracellularly into mono- and disaccharides that are then transported into the cell for further metabolism, and can engage in cross-feeding interactions.67 Among Bifidobacterium species, B. infantis is known to degrade all HMOs present in breast milk, a capability attributed to its wide genetic repertoire of GH enzymes, which work intracellularly.68 In contrast, B. bifidum strains are moderately efficient at HMO degradation and typically employ extracellular degradation.69–72 B. breve strains employ intracellular degradation, and their HMO-degrading capacity is strain-specific, with most strains being able to degrade only non-fucosylated HMOs.71,73 A similar strain variability has been described for B. longum,74,75 with most strains not being able to degrade fucosylated or sialylated HMOs.69,71,75
Bacteroides (e.g., B. fragilis, B. thetaiotaomicron) and Phocaeicola species (e.g., P. vulgatus, P. dorei), metabolize HMOs through a mucin-glycan degradation pathway.76–78 Unlike infant-type bifidobacteria, which specialize in efficient HMO utilization but not mucin glycans, these species are considered generalists in HMO utilization. The specificity of bifidobacteria confers them a selective advantage over Bacteroides species when directly competing in HMO degradation.
Nutrition
The gut microbiome plays an essential role in nutrition, predominantly providing energy and micronutrients locally to the host tissue of the GI tract. The most abundant products of microbial metabolism are SCFAs, which are produced from bacterial fermentation of non-digestible dietary substrates, also known as microbiota-accessible carbohydrates.79 Concentration of each SCFA varies widely between individuals, depending on the diet and age.64 SCFAs are hallmarks of gut health because of their various mechanisms, such as regulating intestinal pH to inhibit the proliferation of pH-sensitive pathogenic bacteria, supplying energy to intestinal epithelial cells, fortifying gut barrier integrity, and mitigating inflammatory responses.80–83 The most abundant SCFA is acetate, typically produced in a 3:1:1 ratio relative to propionate and butyrate, respectively. During the early months, infant-related bifidobacteria that metabolize HMOs, such as B. infantis, B. longum and B. breve, are the major producers of acetate.64,84 Butyrate and propionate levels start to increase at 8 and 10 months of age, respectively.64 Tusukuda et al. hypothesize that this increase is driven by breastfeeding cessation, as reduced lactoferrin and increased free iron may drive these changes, though this was not explored further.64 This increase in butyrate levels is correlated with an increase in butyrate-producing bacteria such as Faecalibacterium prausnitzii and Roseburia species.64 Gut microbes also synthesize B vitamins and vitamin K2, which are essential for various physiological processes, including energy metabolism, nerve function, and bone health.85,86 Compared to 4- and 12-month-olds, the newborn’s gut microbiome has a higher capacity for production of vitamins K2, B6, B7, and B9 (folate). On the other hand, genes for synthesis of vitamins B1, B5, and B12 increase with age.13 Vitamin K2 is exclusively produced by microbes where different isoforms are produced by Escherichia coli, Eggerthella lenta, Veillonella spp., and Bacteroides spp.86 Its production in the infant gut (measured via stool) is influenced by factors such as delivery mode and feeding method, as these directly affect the composition and proportion of K2-producing bacteria in the gut.87
Colonization resistance
The gut microbiota plays an important role in resisting the invasion of foreign pathogens and controlling the proliferation of resident pathobionts, a critical function known as colonization resistance. This resistance is vitally important in newborns who have immature immune systems.88 In the healthy infant gut microbiome, colonization resistance is primarily orchestrated by Bifidobacterium species and pH regulation. Specifically, B. infantis has demonstrated remarkable efficacy in metabolizing HMOs from breast milk, leading to the production of lactate and acetate.89 These SCFAs play a pivotal role in lowering the gut pH, creating an environment unfavorable for the growth of pathogenic bacteria.90 For example, in vitro experiments have shown that acetate production by Bifidobacterium contributes to the prevention of translocation of E. coli O157:H7 Shiga toxin from the gut lumen into the bloodstream.91 Moreover, cross sectional studies have shown that supplementation of breastfed infants with B. infantis significantly reduces the gut pH, the levels of pathogenic bacteria (e.g. Haemophilus influenzae, Escherichia coli, Streptococcus mitis), and the number of genes coding for virulence factors and antibiotic resistance.89,92–95 Supplementation of preterm infants with Bifidobacterium animalis subsp. lactis and with different combinations of Bifidobacterium and Lactobacillus species reduces severe necrotizing enterocolitis, which is caused by invasion of opportunistic pathogens into host tissue.96 Beyond pH regulation, other mechanisms of colonization resistance mediated by Bifidobacterium involve the secretion of bacteriocins and transformation of primary into secondary bile acids which inhibit the growth of pathogenic bacteria.97–99
Immune education
Recent studies highlight a critical early-life period where microbe-host interactions are key to optimal immune cell development and education. In a longitudinal study comparing preterm and full-term infants, Olin et al. observed that immune phenotypes diverged in the first months of life but converged by three months.88 During this window of time, notable changes occurred in immune cell populations, cytokines profiles, and gene expression in both preterm and full-term infants, suggesting that microbial interactions at the gut mucosa are important in shaping this development. Further, the study indicates that the subset of infants experiencing early gut irregularity in their gut microbiome – defined by an extremely low level of Shannon diversity and near complete-dominance of Gammaproteobacteria or other atypical groups – display perturbations of immune system development. Specifically, these included increased expression of the microbial-induced Fc receptor PIgR, expansion of CD8+ T cells that interact with mucosal bacteria, and greater variability in immune cell populations88 This suggests that early imbalances in the gut microbiome disrupt the standard and ideal development of the immune system. Giving further mechanistic evidence for this, Henrick et al. studied a large longitudinal cohort and found that the abundance of HMO-degrading Bifidobacteriaceae in early life significantly affects immune development.100 Low levels of Bifidobacterium correlated with increased immune activation and higher levels of pro-inflammatory cytokines, whereas higher levels were associated with anti-inflammatory and regulatory immune cells and cytokines. Specifically, an exclusively microbial metabolite, indole-3-lactic acid, produced by select bifidobacteria was identified that influences immune-microbe interactions by dampening inflammatory responses, in particular Th2- and Th17-type responses in favor of Th1 and regulatory T cells. Other microbial metabolites like butyrate also promote regulatory T cell (Treg) generation, creating an immune environment conducive to tolerance and decreasing the likelihood of allergic responses.101
What affects the early life gut microbiome?
A multitude of factors affect the composition and functionality of the gut microbiome, and may thus subsequently influence an individual’s health trajectory. In this section, we will focus on five main factors commonly encountered in early life that directly impact the microbiome: delivery mode, antibiotic use, breastfeeding, maternal diet, maternal health and gestational age, and the use of prebiotics/probiotics (Figure 2).
Figure 2.
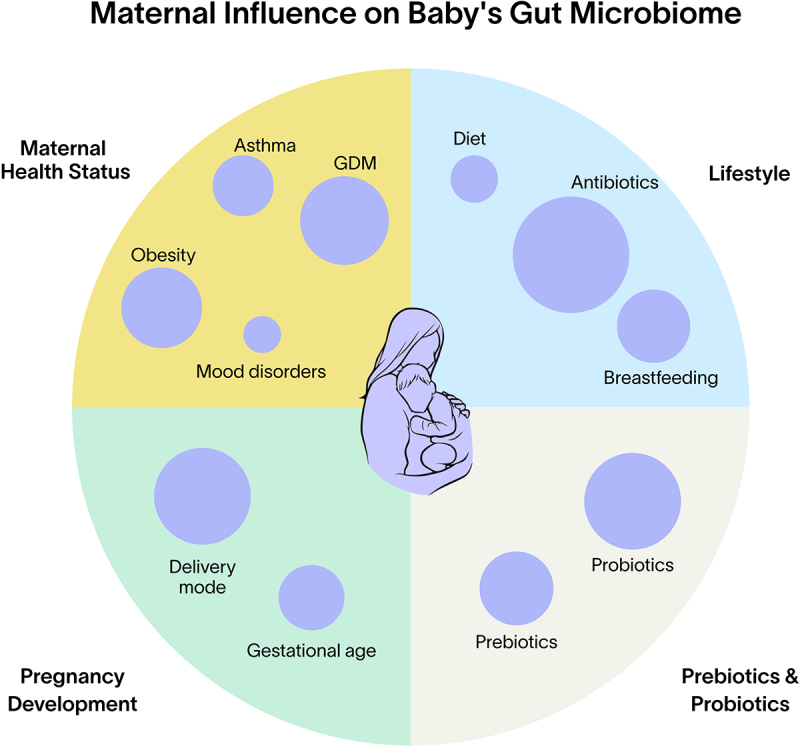
Factors influencing the development of the infant microbiome. GDM = gestational diabetes mellitus, ABX = antibiotic treatment.
Delivery mode
The mode of delivery has a profound impact on the initial colonization and subsequent development of the infant gut microbiome. Vaginal delivery exposes the newborn to the mother’s vaginal and gut microbiota, primarily colonizing the infant’s gut with beneficial bacteria such as Bifidobacterium, Bacteroides, Lactobacillus, and Clostridium species.10 In contrast, infants delivered by C-section are more likely to be colonized by bacteria from the mother’s skin and the hospital environment, including Staphylococcus, Streptococcus, and Clostridioides difficile, as well as other opportunistic pathogens from the Enterobacteriaceae family.10,11
Cesarean delivery has been associated with a delayed colonization of the gut by beneficial bacteria, particularly those in the Bacteroidota phylum, and a lower overall microbial diversity.48,49,102–104 While some studies have shown that these differences can persist for up to two years,48,49 others show partial or almost complete recovery of the gut microbiome by 6 months of age.102–104 This disturbance in early microbiome development has been suggested to contribute to the increased risk of immune-mediated conditions such as asthma, allergies, obesity, and other chronic diseases seen in C-section born children later in life.33,105,106 Even though the gut microbiome of C-section-born infants may eventually resemble that of vaginally delivered infants, the existence of a critical early-life window of immune system maturation suggests that initial microbial imbalances may have long-term consequences“s.4,41
Antibiotic exposure
Antibiotic exposure during the prenatal and neonatal periods can significantly impact the infant’s microbiome. Use of intrapartum antibiotics have been shown to reduce the amount of species transmitted from mothers to infants,107 and prenatal and intrapartum antibiotic use has been associated with a marked decrease in the abundance of Bifidobacterium and Bacteroides, and an increase in the abundance of members of the Enterobacteriaceae family, and Enterococcus and Clostridium species.12,108–110 Antibiotics have also shown to increase the abundance of antibiotic resistance genes in the infant’s gut microbiome.109,111
Neonatal antibiotic use can delay the maturation of the gut microbiome and cause a potential regression relative to its expected developmental trajectory, with a marked decrease in Bifidobacterium species and an increase in members of the Enterobacteriaceae family.31,48,112 In addition, the gut microbiota of antibiotic-exposed infants exhibits less strain diversity compared to non-exposed infants,113 which could impact the functional capacity of their gut microbiome. In some infants, these microbiome imbalances can persist long-term.22 Finally, antibiotic exposure has been linked to an increased likelihood of adverse health outcomes, including higher odds of atopic dermatitis (AD), asthma, obesity, and food allergies.114,115
Infant diet
As discussed above, breastfeeding profoundly affects the development of the infant gut microbiome.13,116–118 It promotes the dominance of beneficial Bifidobacterium species, such as B. infantis, B. breve, B. bifidum, and B. longum, which can degrade HMOs.7,12,13,119 These HMOs, along with other bioactive components in breast milk like IgA and lactoferrin, enhance mucosal immunity and inhibit pathogen colonization.58,120,121 In many Cesarean-delivered infants breastfeeding also counteracts imbalances in the gut microbiota by replenishing levels of bifidobacteria and promoting a microbial composition more similar to that of vaginally-delivered infants.13,117 In contrast, formula-fed infants often have lower levels of Bifidobacterium species13,116 and higher levels of opportunistic pathogens117,122 potentially due to absence of key bioactive components present in breast milk.116,118,123
The introduction of solid foods, typically around 6 months, marks a transformative phase in the development of the infant gut microbiome, diversifying the microbial composition and enhancing the functional capacity to metabolize new substrates like carbohydrates, fibers, and proteins.55,64 Dietary diversity positively correlates with Bifidobacterium abundance,54 but the timing of solid food introduction also shapes microbiota composition. Parkin et al. found that breastfed infants introduced to solids before 5 months had increased microbiome diversity, including higher levels of B. infantis.124 Similarly, Differding et al. reported that early complementary feeding (≤3 months) increased diversity and butyrate-producing taxa such as Lachnospiraceae and Roseburia by 12 months, alongside elevated SCFA concentrations.125
Maternal diet
The overall impact of maternal diet on the infant microbiome is an emerging topic of study. However, there is clear evidence linking maternal consumption of fruits and vegetables during pregnancy to the composition of the infant gut microbiome. At one month, infants born to mothers who ate more vegetables during pregnancy tended to have a lower Shannon diversity and a higher relative abundance of Bifidobacterium.19 Conversely, a lower intake of these foods was associated with an increase in microbial taxa such as Sutterella, Prevotella, Lachnoclostridium, and Flavonifractor at two months.21 Additionally, a diet high in fat during pregnancy resulted in lower levels of Bacteroides at six weeks of age.20 This reduction appears to stem from a decreased Bacteroides abundance in the maternal gut microbiota, limiting vertical transmission during delivery, or due to an increased likelihood of cesarean delivery, associated with high-fat diets. Maternal diet also influences breast milk composition, including HMOs and other bioactive molecules that shape the infant gut microbiome.126 For instance, Neu5Gc from red meat is incorporated into HMOs and associated with increased Bacteroides abundance in infant stool.126
Maternal health and gestational age
Conditions like gestational diabetes (GDM) and obesity are linked to the maternal microbiome, and in turn also linked to microbial composition of the infant and its future health risks.127–129 Infants born to mothers with GDM are more likely to develop obesity and metabolic diseases in later life.129,130 Maternal obesity has also been associated with differences in the infant microbiome, including higher levels of Bacteroides.131–133 However, disentangling direct microbiome effects from confounding lifestyle factors – such as shared dietary patterns, activity levels, breastfeeding duration, dietary transitions, and the introduction of complementary foods – is challenging.131,133
In addition, maternal mood disorders, asthma, human immunodeficiency virus, Group B Streptococcus (GBS), eczema, and inflammatory bowel disease (IBD), have all been linked to variations in the infant’s gut microbiome.36,134 For example, maternal prenatal stress was associated with an increased abundance of Proteobacteria in infants which may be linked to inflammation.134 Reduced levels of beneficial bacteria Lactobacillus and Bifidobacterium in infants was associated with maternal stress134 as well as maternal asthma.36
Finally, gestational age plays a role in affecting the microbial landscape of the neonatal gut. Premature infants, defined as those born before 35 weeks of gestation, typically exhibit a microbiota characterized by less diversity than term infants, fewer bacterial species, and a distinct profile that can persist for up to four years.15,135 These infants often experience delayed colonization by beneficial microbes such as Bifidobacterium, which dominate vaginally delivered term infants, and display an increased presence of opportunistic pathogens such as Staphylococcus, Enterococcus and Enterobacter.16 One study also observed markedly reduced SCFAs concentrations in preterm infants compared to full term infants, primarily due to lower acetate levels.14 These reduced levels reflect gut immaturity, with limited SCFA-producing bacteria potentially due to reduced exposure to maternal microbiota and HMOs and/or increased antibiotic use in preterm infants136
Prebiotics and probiotics
Prebiotic and probiotic supplementation during and after pregnancy may contribute to better outcomes in certain conditions or specific instances. For example, supplementation with Lactobacillus rhamnosus GG strain during pregnancy was reported to promote the colonization of Bifidobacterium in the infant gut.137,138 Supplementing infants with different strains of Bifidobacterium infantis or a mix of Bifidobacterium and Lactobacillus is effective in increasing gut bifidobacteria levels when paired with breastfeeding to supply the HMOs that feed the bifidobacteria. This supplementation also helps lower gut pH and enhance the production of SCFAs.92,95,139
Probiotics have shown success in restoring the microbial and functional profile of infants born by C-section to one more similar to those born vaginally. A study by Gong et al. found that a two-week supplementation of infants born by C-section with a probiotic mix containing B. longum, L. acidophilus, and E. faecalis promoted the growth of beneficial bacteria such as Bacteroides, Veillonella, and Faecalibacterium while reducing the levels of Klebsiella.140 Korpela et al. found that supplementing with a mixture of B. breve, Propionibacterium freudenreichii, and L. rhamnosus during pregnancy and to babies up to 6 months, increased the abundance of genes associated with HMO degradation in both vaginally and C-section-born infants.141 This effect may be exclusively attributed to B. breve, which is a specialized HMO degrader.141 Probiotics have also shown promising effects on the gut microbiome of preterm infants. Supplementing with a mixture of Bifidobacterium and Lactobacillus strains lead to a community that resembles the gut microbiome of full-term infants.142,143
Supplementing infant formula with HMOs has been shown to shift the gut microbiome toward a composition similar to that of breastfed infants by promoting the growth of Bifidobacterium and reducing levels of opportunistic pathogens like Clostridioides difficile.144–146 Similar benefits have been observed when supplementing infant formula with probiotics or symbiotics.147–149 Lagkouvardos et al. showed that a formula supplemented with Lactobacillus fermentum and galactooligosaccharides increased the levels of Bifidobacterium and Lactobacillaceae, while decreasing Blautia, Ruminococcus gnavus, and butyrate levels at 4 months of age.148 However, the study did not provide species-level data for Bifidobacterium, making it unclear whether the increases were specific to infant-associated species or included other non-infant-associated species.
Studies have also focused on the effects of probiotics in preventing chronic conditions in high-risk infants. Prenatal supplementation of women with obesity using a Bifidobacterium and Lactobacillus probiotic has been shown to reduce Collinsella —a genus associated with obesity150— and increase Akkermansia in their infants, potentially redirecting their gut microbiome to a profile not associated with obesity.151 Additionally, probiotics – alone or in combination with prebiotics – given to both pregnant mothers and infants have been linked to a reduced risk of developing eczema.152,153
These findings suggest that prebiotics and probiotics, specifically particular strains, can be a promising synergistic approach to fostering the growth of beneficial gut bacteria, supporting microbiome development in preterm infants, and restoring the gut microbiome of infants born by C-section or vaginally born infants that have not been exposed to beneficial microbes. Thus, early supplementation could help alleviate a range of pediatric health issues, including metabolic disorders, allergies, and autoimmune diseases.
How does early life gut microbiome affect disease development?
As we have summarized above, the gut microbiome affects many physiological, metabolic, and immune processes.118,154 In this section we will review how disturbance of these processes in early life may contribute to disease in later life.
Allergic disease and asthma
The rising prevalence of allergies among children has propelled research pointing to a complex relationship between genetic predispositions and multiple environmental factors.155 Notably, many of these environmental influences – including antibiotic use, Cesarean delivery, and dietary habits – can modulate the gut microbiome. Thus, the gut microbiome may contribute to the development of allergic conditions, which often follow a trajectory from atopic dermatitis (AD) and food allergy to asthma and rhinitis, a progression commonly referred to as the atopic march.155
AD often emerges in infancy, affecting an estimated 16.5% of children in the US.156 Although AD may resolve with age, children with a persistent phenotype have a 4-fold and 7-fold higher risk of developing asthma and allergic rhinitis, respectively.157 The gut microbiome has been associated with the development of AD, though a causal relationship has not been firmly established. Infants younger than 6 months with AD often exhibit high levels of Akkermansia.158,159 However, it is unclear if Akkermansia species contribute to AD pathogenesis or serve as disease-associated biomarker, as A. muciniphila has been described to help maintain intestinal integrity160,161 and has been linked to protective effects against metabolic syndrome.162–164 High levels of A. muciniphila may represent a host immune response to an inflammatory event rather than a direct causal factor in AD pathogenesis.165 Additionally, high levels of Enterobacteriaceae, particularly Klebsiella pneumoniae, are found in infants with AD from birth to 3 months.166 At one week of age, infants born by C-section who later develop eczema, have higher levels of Escherichia, Citrobacter, and Enterobacter than infants who do not develop the condition.167 Conversely, infants from 3 weeks to 12 months with AD show low levels of the Erysipelotrichaceae family166,168 and butyrate-producing Faecalibacterium,158,166,169 a taxa associated with anti-inflammatory properties. Related to this, infants with lower levels of fecal butyrate at 3 and 6 months had an increased risk of developing AD up to 8 years of age.170 These patterns suggest a potential causal role for the gut microbiome in AD, but whether these taxa are contributors to the disease or predictive biomarkers requires intervention studies to address.
Food allergies affect approximately 6% of children171 and 8% of adults in the US.172 IgE-mediated food allergies often follow eczema and can manifest within an infant’s first year, typically around 6 months when solid foods are introduced. Food allergies are driven by a complex interplay of genetics and environmental factors.173 However, recent research also uncovered a direct link between the early gut microbiome composition and IgE-mediated food allergies.174,175 For example, one study showed that mice that were colonized by bacteria from infants with cow’s milk protein allergy (CMPA) exhibited severe allergic reactions to cow’s milk, while those colonized by bacteria from healthy infants did not.175 Supporting this, a longitudinal study found that infants with elevated levels of Clostridia and Firmicutes at 3 and 6 months of age were more likely to outgrow milk allergy later in childhood.176 The gut microbiome also regulates food allergy-related immune responses through the induction of Tregs, which are essential for suppressing hypersensitive immune reactions. In murine models, introducing a defined consortia of Clostridia species induced Tregs expressing the transcription factor ROR-γt in a MyD88-dependent manner, effectively mitigating food allergy symptoms.174
Human infants born via C-section are at increased risk of developing food allergies later in life,105,177–179 perhaps because they lack the diverse protective bacteria acquired during vaginal birth.13,42,48,106,180 While distinct gut microbiome changes have been observed in cohorts with various food allergies (such as egg, peanut, soy, wheat, and milk), inconsistency across studies complicates interpretation.174,176,179,181
Asthma affects approximately 8.1% children in the US,182 and the gut microbiome in early infancy has been closely linked to its development. Children at high risk were found to exhibit delayed gut microbiome diversification in the first year of life.183 Delayed microbiome maturation – the process of the microbiome changing with age – at 1 year of age has been associated with asthma development at age 5.106 Compared to vaginally-born babies, those born via C-section face twice the risk of developing asthma,105 but this risk can be mitigated if their gut microbiome reaches appropriate maturation by age 1.184 However, those children retaining a C-section gut microbiome signature at age 1 have three times the asthma risk by age 6 compared to C-section babies without the signature. The main involved taxa are again bifidobacteria as low levels of Bifidobacterium species at 1 month, 3 months, and 1 year of age have been associated with an increased risk of developing asthma by 5 years of age.29,106,185 In animal models of asthma, all three main SCFAs186,187 have been shown to protect against airway inflammation, and this is attributed to the stimulation of specific immune cell subsets capable of preventing T helper 2 immune responses.100
Metabolic diseases
Children born to mothers with obesity have a higher risk of developing obesity later in life.127,133 This connection is further supported by the description of microbes connected to obesity passed down from mother to child at birth.127,133 Additionally, a reduction in butyrate and other SCFA-producing bacteria in newborns from mothers with obesity has been linked to an increased risk of obesity but the mechanism here is not clear.131,133
Autoimmune disease
The early-life development of the gut microbiome has also been linked with Type 1 diabetes (T1D).31,188,189 SCFA-producing bacteria, such as Bifidobacterium and Lachnospiraceae, are consistently reduced in T1D patients. A decrease in SCFAs may contribute to increased intestinal permeability and bacterial antigen exposure, potentially contributing to an undesired immune response that may lead to autoimmunity.
Inflammatory Bowel Disease (IBD), encompassing Crohn’s disease and ulcerative colitis, can already manifest in childhood. Studies have shown a reduction in bacterial diversity and alterations of specific bacterial taxa in the gut of children with IBD, but this could be driven by the high level of existing inflammation.9,118 As in T1D, the microbial imbalance associated with triggering IBD is thought to involve a compromised intestinal barrier, which allows bacteria to penetrate the mucosal layer and activate damaging immune responses, thereby triggering an underlying genetic predisposition. Longitudinal microbiome studies following infants from early life until developing IBD do not exist and will be required to establish whether early life microbiome disruptions predispose to it.
Enhancing the methodological approaches in gut microbiome studies
The previously reviewed studies demonstrate compelling evidence for a link between the gut microbiome and various health outcomes, although substantial work remains to be done in this field. To fully elucidate the role of the gut microbiome in these diseases, robust and comprehensive research methodologies have to be followed starting from proper human studies. Although significant progress has been made, current microbiome studies often face limitations such as small sample sizes, cross sectional vs longitudinal sampling, lack of household controls, minimal clinical data collection and outdated and narrow sequencing approaches. In this section we will review these shortcomings and suggest how they can be addressed.
Sample methodology
Standardized sample collection and storage are essential for reliable microbiome research. Variability in protocols, including differences in storage conditions and processing times, can obscure biological differences. Prompt stabilization, through immediate cooling and storage at − 80°C, or using validated preservation methods such as desiccation or stabilization buffers are crucial to maintain sample integrity,190 as rapid compositional changes occur at room temperature.191 In addition, using DNA extraction methods with minimal levels of bias is crucial to perform accurate microbiome analysis.
Larger and more diverse cohorts
Few studies on the infant microbiome involve larger cohorts and many studies are based on small to medium-sized cohorts (fewer than 400 participants), which limits statistical power and the ability to detect subtle differences and meaningful associations.192 Moreover, larger cohorts can better capture the diversity of the human population, addressing the disparity in microbiome research by encompassing various ethnicities and their genetic backgrounds, environmental variables, and lifestyle factors.193 In the context of the early life gut microbiome, capturing such diversity is crucial, as we have seen early microbial colonization and development are highly influenced by these factors.
Longitudinal sampling
Increased sampling frequency enables researchers to better understand the temporal dynamics of the gut microbiome. Individuals seem to differ in the rate of change of their gut microbiomes, even over short time scales, suggesting that characterizing temporal variability is crucial for understanding individual microbiomes.194,195 In the context of the infant gut microbiome, high-frequency sampling is particularly valuable for tracking microbial development and maturation through critical early life stages.7 Understanding these temporal dynamics simplifies the identification of connections between microbial species and health outcomes, facilitating the interpretation of correlations among taxonomic groups.196 However, longitudinal microbiome data analysis remains challenging and the field could benefit from improved statistical methodologies.
Multiple samples per family
Collecting microbiome samples from multiple family members offers the opportunity to dissect the contributions of genetic and environmental factors to the gut microbiome.197,198 Tavalire et al. demonstrated that while environmental factors account for most of the variance in microbial composition among family members, genetic similarity more frequently explains variance in microbial abundance than does a shared home environment.199 Similarly, Xie et al. found that microbial profiles of twin pairs living in different regions differed more than those of twins living in the same region, suggesting a significant environmental influence.200 However, their study also revealed that genetic factors play an important role in determining microbial abundance. Notably, they observed that the variation in the abundance of certain bacterial groups, such as Dorea, Prevotella, and Oxalobacter, is predominantly influenced by genetics rather than environmental factors.
Detailed survey data on health and lifestyle
Integrating detailed survey data on infants’ health, diet, medication use, and lifestyle provides valuable context to microbiome data. Such comprehensive metadata enables more nuanced analyses, helping to identify specific factors that influence the gut microbiome and facilitate the removal of microbiome variability that is due to confounding factors such as diet, medication use, other diseases, etc. A recent large-scale study by Gacesa et al. illustrates the potential of gathering extensive metadata. Their analysis of the gut microbiome of 8,208 Dutch individuals from a three-generational cohort, encompassing 2,756 families, included more than 200 host and environmental factors.201 These factors include detailed information on physical and mental health, medication use, dietary habits, socioeconomic status, and both childhood and current exposome. This comprehensive metadata collection enabled researchers to identify more than 2,500 associations between the microbiome and health, providing a thorough analysis of how lifestyle, diet, and health parameters influence the gut microbiome.
Sampling before disease onset
Collecting microbiome samples before the onset of disease offers significant methodological advantages for gut microbiome research by allowing researchers to establish temporal relationships between microbiome changes and disease development. It also removes variability that is an indirect effect of the disease rather than contributing to the disease. The hallmark TEDDY study exemplifies this approach, focusing on the onset and development of T1D and its relationship with the gut microbiome.31 By collecting stool samples monthly from children starting at three months of age until T1D diagnosis, researchers showed the potential protective effect of SCFAs in early-onset T1D and identified microbial biomarkers of disease development. Healthy controls had higher levels of Streptococcus thermophilus and Lactococcus lactis while cases had more Bifidobacterium pseudocatenulatum, Roseburia hominis, and Alistipes shahii.31 These biomarkers can identify individuals at risk of developing T1D before clinical symptoms appear, facilitating early intervention and potentially preventing disease onset.
Comprehensive functional analysis
While much of the current research focuses on the taxonomic composition of the gut microbiome, it is increasingly evident that microbial functions are equally, if not more, crucial for understanding the microbiome’s role in health and disease.202 To embrace the idea that the microbiome is better described through its functions than its composition, we advocate for the widespread adoption of shotgun metagenomics in microbiome studies. Unlike 16S rRNA sequencing, which has limited taxonomic resolution and cannot perform functional analysis, shotgun metagenomics assesses the functional capacity of microbial communities. This approach enables researchers to infer how microbial communities may contribute to host metabolism, immune function, and disease.203 This approach offers a more comprehensive understanding of the microbiome’s role in health, particularly in identifying the functional capacity that may affect host physiology. However, to determine what microbes are actively doing, additional techniques like transcriptomics and metabolomics should be used, but these come with additional requirements on sample collection. By integrating functional analysis into microbiome research, we can move beyond simply identifying “who is there” to understanding “what they are doing,” ultimately leading to more targeted and effective interventions for promoting health and preventing disease.
Does an ideal early life gut microbiome exist?
The concept of an “ideal” early-life gut microbiome is nuanced and complex, considering the multitude of factors that shape its development from birth. Due to this complexity, defining a universal “healthy” microbiome is challenging. In both adults and infants, the variability in microbiome composition across individuals is vast. While the core functional roles are conserved in adults,202 it remains to be explored whether the same is true in infants. We propose that a healthy gut microbiome can be defined as one that complements the individual’s lifestyle, supporting functional processes that maintain health and not contribute to development of disease.
More specifically, we believe that a healthy or ideal microbiome should be defined by function. As discussed above the core functions of an early life microbiome involve digestion of carbohydrates that are inaccessible to the host, immune system maturation, and colonization resistance. An obvious example of a crucial digestion function is breakdown of HMOs. Evolutionary pressure on the mother must have been remarkable in order to secrete vast quantities of HMOs into breast milk, which are digestible only by specific microorganisms in the gut. It would thus be a stretch to argue that not having HMO-digesting microbes is fine, or even ideal. Linked to immune system maturation, the microbiome literature points to the crucial role of bifidobacteria,100 but also to the presence of keystone adult taxa in early life, such as Akkermansia and Faecalibacterium species (at low relative abundance). Presence of these adult taxa is associated with not developing allergic and atopic conditions.29,119,158,204 The final core function is colonization resistance. Microbes that contribute to colonization resistance of pathogenic microorganisms should be considered part of an ideal microbiome because they prevent GI infection which is a high-risk condition for infants.
We are of the opinion that it will eventually become standard of care to test early life microbiome composition and employ clinically actionable interventions for specifically optimizing these core functions in only those infants that could benefit from them. Before this can happen many questions have to be answered, including additional validation of technology and interventions, and the updated regulatory frameworks under which this will take place. While we are of the opinion that testing and interventions are important, we do not advocate for universal probiotic or prebiotic supplementation. Instead, supplementation should be approached on a case-by-case basis, guided by a do-no-harm principle, and considered only for infants whose microbiome lacks essential functions. For infants whose microbiome already includes core functions, supplementation is not necessary and may have unintended consequences.
Besides supplying targeted prebiotics and specific strains of probiotics directly to infants, a variety of other strategies can be employed to optimize early-life microbiome development. These include maternal tailored nutritional interventions during pregnancy, sustained breastfeeding practices, and the use of prebiotics and probiotics by the mother during pregnancy and lactation. Additionally, infancy-specific interventions, such as introducing a diverse range of solid foods at the appropriate age, ensuring minimal exposure to unnecessary antibiotics, fostering a healthy but microbially rich environment, may further support healthy microbiome development (Table 1). These strategies emphasize the importance of personalized approaches to microbiome health, recognizing the unique microbiome and environmental contexts of each infant.
Table 1.
Strategies for supporting healthy microbiome development in infants.
| Target | Description |
|---|---|
| Maternal Diet and Nutrition | Education about the importance of maternal diet during pregnancy can impact the infant’s gut microbiome. A healthy diet can support the development of beneficial microbial communities. |
| Infant feeding | Breastfeeding introduces beneficial bacteria and provides HMOs, promoting the growth of beneficial gut flora. Education can extend breastfeeding duration, benefiting both mothers and infants. When breastfeeding is not possible, a formula containing HMOs or adding an HMO supplement is advisable. |
| Prebiotics and Probiotics | Using prebiotics and specific strains of probiotics during pregnancy and lactation can alter the infant’s gut microbiome. These interventions can significantly influence the microbial composition during critical developmental periods, potentially impacting the child’s long-term health and immune function. |
| Antibiotic Use | Educating on the risks of antibiotic overuse, especially during key developmental phases of the infant’s microbiome, can lead to more judicious use, such as avoiding antibiotics for viral infections where they are ineffective. Additionally, when antibiotics are necessary, it’s crucial to take steps to protect and restore beneficial microbes, as some may never fully recover post-treatment. |
| Solid Foods Introduction | Timely and diverse introduction of solid foods influences the gut microbiome’s complexity and diversity, impacting overall health. Education on the timing and types of foods can optimize microbiome development. |
| Education on Birth Mode | Informing expectant parents and healthcare providers about the impact of C-section versus vaginal birth on the microbiome can lead to more natural birth practices when advisable, supporting optimal microbiome development. The option of vaginal seeding after appropriate testing of the vaginal microbiome and the use of antibiotics before or after cord clamping during a C-section birth could also be presented to parents, although research on the benefits is not conclusive. |
| Microbiome-friendly environment | Educating on how harsh cleaning products and over-sanitization can negatively impact microbiome diversity and richness, while exposure to natural environments, pets, and farm animals do the opposite. |
Conclusion
We have reviewed the role of the early life gut microbiome and its role in shaping health trajectories from early infancy through childhood. We conclude that the highly dynamic interaction between the gut microbiome and host is critical for physiological development and disease resilience. Each phase during the first three years of life influences the early-life gut microbiome, from prenatal influences, where maternal diet, health, and antibiotic usage shape the newborn microbiome, to the impact of birth factors such as mode of delivery and antibiotic administration, to postpartum factors, particularly breastfeeding and the introduction of prebiotics and probiotics. These colonization events and associated effects on the infant’s immune system are associated with long-term impacts on immune function, metabolic processes, and overall health, impacting the risk for chronic conditions such as allergies, autoimmune diseases, and metabolic disorders.
The understanding of the various factors influencing the early life microbiome highlights the possibility of targeted strategies aimed at optimizing gut microbiota composition from birth. Such strategies hold promise to improve health outcomes, particularly in mitigating risks associated with cesarean delivery, prematurity, and formula feeding. Targeted interventions such as probiotics and specific strains of prebiotics, when applied during critical windows of immune and metabolic development, may affect health well into adulthood.
In conclusion, the complexity of the early life gut microbiome and its substantial influence on health across the lifespan calls for a continued focus on understanding this dynamic ecosystem. As the research field matures, it is critical to improve our studies and processes to translate these findings into public health strategies and work toward clinical actionability. Such strategies promise not only to enhance immediate well-being in infancy but also to pave the way for a healthier future generation.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
Dr. Harold Nunez, Dr. Pamela A. Nieto, and Dr. Kimberley Sukhum are employees of Tiny Health, a company that provides microbiome testing throughout various life stages.
Dr. Ruben A. Mars is a founding advisor at Tiny Health. This position is approved by the Medical-Industry Relations Committee of Mayo Clinic but fully independent of his employment at Mayo Clinic.
Cheryl Sew Hoy is the founder and CEO of Tiny Health.
Tiny Health offers microbiome testing that is processed in a CLIA and CAP-certified lab, with a focus on providing wellness-related insights into the human microbiome.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study
References
- 1.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4). doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson RC, Manges AR, Finlay BB, Prendergast AJ.. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol. 2019;27(2):131–24. doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Ryan CA, Boyaval P, Dempsey EM, Ross RP, Stanton C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020;28(1):28–45. doi: 10.1016/j.tim.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Xiao L, Zhao F. Microbial transmission, colonisation and succession: from pregnancy to infancy. Gut. 2023;72(4):772–786. doi: 10.1136/gutjnl-2022-328970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox TO, Lundgren P, Nath K, Thaiss CA. Metabolic control by the microbiome. Genome Med. 2022;14(1):80. doi: 10.1186/s13073-022-01092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 9.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, Sears MR, Mandhane PJ, Turvey SE, Subbarao P, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 13.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Dahl C, Stigum H, Valeur J, Iszatt N, Lenters V, Peddada S, Bjørnholt JV, Midtvedt T, Mandal S, Eggesbø M. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int J Epidemiol. 2018;47(5):1658–1669. doi: 10.1093/ije/dyy064. [DOI] [PubMed] [Google Scholar]
- 15.Fouhy F, Watkins C, Hill CJ, O’Shea C-A, Nagle B, Dempsey EM, O’Toole PW, Ross RP, Ryan CA, Stanton C. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi: 10.1038/s41467-019-09252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpela K, Blakstad EW, Moltu SJ, Strømmen K, Nakstad B, Rønnestad AE, Brække K, Iversen PO, Drevon CA, de Vos W. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. 2018;8(1):2453. doi: 10.1038/s41598-018-20827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feehily C, O’Neill IJ, Walsh CJ, Moore RL, Killeen SL, Geraghty AA, Lawton EM, Byrne D, Sanchez-Gallardo R, Nori SRC, et al. Detailed mapping of Bifidobacterium strain transmission from mother to infant via a dual culture-based and metagenomic approach. Nat Commun. 2023;14(1):3015. doi: 10.1038/s41467-023-38694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yassour M, Jason E, Hogstrom LJ, Arthur TD, Tripathi S, Siljander H, Selvenius J, Oikarinen S, Hyöty H, Virtanen SM, et al. Strain-Level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host & Microbe. 2018;24(1):146–154.e4. doi: 10.1016/j.chom.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrera-Rubio R, Pickett-Nairne K, González-Solares S, Collado MC, Venter C. The maternal diet index and offspring microbiota at 1 month of life: Insights from the Mediterranean birth cohort MAMI. Nutrients. 2024;16(2):314. doi: 10.3390/nu16020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan H-Y, Tung Y-T, Yang Y-C, Hsu JB, Lee C-Y, Chang T-H, Su E-Y, Hsieh R-H, Chen Y-C. Maternal vegetable and fruit consumption during pregnancy and its effects on infant gut microbiome. Nutrients. 2021;13(5):1559. doi: 10.3390/nu13051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparrini AJ, Wang B, Sun X, Kennedy EA, Hernandez-Leyva A, Ndao IM, Tarr PI, Warner BB, Dantas G. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat Microbiol. 2019;4(12):2285–2297. doi: 10.1038/s41564-019-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kortman GAM, Timmerman HM, Schaafsma A, Stoutjesdijk E, Muskiet FAJ, Nhien NV, van Hoffen E, Boekhorst J, Nauta A. Mothers’ breast milk composition and their respective infant’s gut Microbiota differ between five distinct rural and urban regions in Vietnam. Nutrients. 2023;15(22):4802. doi: 10.3390/nu15224802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morandini F, Perez K, Brot L, Seck SM, Tibère L, Grill J-P, Macia E, Seksik P. Urbanization associates with restricted gut microbiome diversity and delayed maturation in infants. iScience. 2023;26(11):108136. doi: 10.1016/j.isci.2023.108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panzer AR, Sitarik AR, Fadrosh D, Havstad SL, Jones K, Davidson B, Finazzo S, Wegienka GR, Woodcroft K, Lukacs NW, et al. The impact of prenatal dog keeping on infant gut microbiota development. Clin Exp Allergy. 2023;53(8):833–845. doi: 10.1111/cea.14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tun HM, Konya T, Takaro TK, Brook JR, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, et al. Exposure to household furry pets influences the gut microbiota of infants at 3–4 months following various birth scenarios. Microbiome. 2017;5(1):40. doi: 10.1186/s40168-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldriwesh MG, Al-Mutairi AM, Alharbi AS, Aljohani HY, Alzahrani NA, Ajina R, Alanazi AM. Paediatric asthma and the microbiome: A systematic review. Microorganisms. 2023;11(4):939. doi: 10.3390/microorganisms11040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darabi B, Rahmati S, HafeziAhmadi MR, Badfar G, Azami M. The association between caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2019;15(1):62. doi: 10.1186/s13223-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56(5):661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark Å, Hagopian WA, Rewers MJ, She J-X, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 33.Scheepers LEJM, Penders J, Mbakwa CA, Thijs C, Mommers M, Arts ICW. The intestinal microbiota composition and weight development in children: The KOALA birth cohort study. Int J Obes (Lond). 2015;39(1):16–25. doi: 10.1038/ijo.2014.178. [DOI] [PubMed] [Google Scholar]
- 34.Di Profio E, Magenes VC, Fiore G, Agostinelli M, La Mendola A, Acunzo M, Francavilla R, Indrio F, Bosetti A, D’Auria E, et al. Special diets in infants and children and impact on gut microbioma. Nutrients. 2022;14(15):3198. doi: 10.3390/nu14153198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Mantrana I, Selma-Royo M, González S, Parra-Llorca A, Martínez-Costa C, Collado MC. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;11(4):962–978. doi: 10.1080/19490976.2020.1730294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, Gordon A. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes. 2021;13(1):1–30. doi: 10.1080/19490976.2021.1897210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2023;7(7):CD005496. doi: 10.1002/14651858.CD005496.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suárez-Martínez C, Santaella-Pascual M, Yagüe-Guirao G, Martínez-Graciá C. Infant gut microbiota colonization: Influence of prenatal and postnatal factors, focusing on diet. Front Microbiol. 2023;14:1236254. doi: 10.3389/fmicb.2023.1236254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24(1):133–145.e5. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podlesny D, Fricke WF. Strain inheritance and neonatal gut microbiota development: A meta-analysis. Int J Med Microbiol. 2021;311(3):151483. doi: 10.1016/j.ijmm.2021.151483. [DOI] [PubMed] [Google Scholar]
- 41.Enav H, Bäckhed F, Ley RE. The developing infant gut microbiome: A strain-level view. Cell Host Microbe. 2022;30(5):627–638. doi: 10.1016/j.chom.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon M-C, Kiilerich P, Akrami R, Krämer M, Uhlén M, et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe. 2021;29(5):765–776.e3. doi: 10.1016/j.chom.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Li L, Jin B, Xu X, Zuo X, Li Y, Li Z. The effects of delivery mode on the gut microbiota and health: State of art. Front Microbiol. 2021;12:724449. doi: 10.3389/fmicb.2021.724449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jokela R, Ponsero AJ, Dikareva E, Wei X, Kolho KL, Korpela K, de Vos WM, Salonen A. Sources of gut microbiota variation in a large longitudinal Finnish infant cohort. EBioMedicine. 2023;94:104695. doi: 10.1016/j.ebiom.2023.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wernroth M-L, Peura S, Hedman AM, Hetty S, Vicenzi S, Kennedy B, Fall K, Svennblad B, Andolf E, Pershagen G, et al. Development of gut microbiota during the first 2 years of life. Sci Rep. 2022;12(1):9080. doi: 10.1038/s41598-022-13009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez-Perez GI, Chen Y, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 50.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: Mom matters. Trends Mol Med. 2015;21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Guglielmo MD, Franke KR, Robbins A, Crowgey EL. Impact of early feeding: Metagenomics analysis of the infant gut microbiome. Front Cell Infect Microbiol. 2022;12:816601. doi: 10.3389/fcimb.2022.816601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, Zhuo N, Wang H, Wang L, Wu D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020;10(1):15792. doi: 10.1038/s41598-020-72635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Zeng S, Egan M, Cherry P, Strain C, Morais E, Boyaval P, Ryan CA, M Dempsey E, Ross RP, et al. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes. 2021;13(1):1–15. doi: 10.1080/19490976.2021.1900996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homann C-M, Rossel CAJ, Dizzell S, Bervoets L, Simioni J, Li J, Gunn E, Surette MG, de Souza RJ, Mommers M, et al. Infants’ first solid foods: Impact on gut Microbiota development in two intercontinental cohorts. Nutrients. 2021;13(8):2639. doi: 10.3390/nu13082639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKeen S, Roy NC, Mullaney JA, Eriksen H, Lovell A, Kussman M, Young W, Fraser K, Wall CR, McNabb WC, et al. Adaptation of the infant gut microbiome during the complementary feeding transition. PLoS One. 2022;17(7):e0270213. doi: 10.1371/journal.pone.0270213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016;16(1):86. doi: 10.1186/s12876-016-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guittar J, Shade A, Litchman E. Trait-based community assembly and succession of the infant gut microbiome. Nat Commun. 2019;10(1):512. doi: 10.1038/s41467-019-08377-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54(20):7471–7480. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 60.Chen X. Human milk oligosaccharides (HMOS): Structure, function, and enzyme-catalyzed synthesis. Adv Carbohydr Chem Biochem. 2015;72:113–190. doi: 10.1016/bs.accb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinleyici M, Barbieur J, Dinleyici EC, Vandenplas Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes. 2023;15(1):2186115. doi: 10.1080/19490976.2023.2186115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao L, van De Worp WR, Stassen R, van Maastrigt C, Kettelarij N, Stahl B, Blijenberg B, Overbeek SA, Folkerts G, Garssen J, et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur J Immunol. 2019;49(7):1001–1014. doi: 10.1002/eji.201847971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sodhi CP, Wipf P, Yamaguchi Y, Fulton WB, Kovler M, Niño DF, Zhou Q, Banfield E, Werts AD, Ladd MR, et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr Res. 2021;89(1):91–101. doi: 10.1038/s41390-020-0852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsukuda N, Yahagi K, Hara T, Watanabe Y, Matsumoto H, Mori H, Higashi K, Tsuji H, Matsumoto S, Kurokawa K, et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021;15(9):2574–2590. doi: 10.1038/s41396-021-00937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362(6418):eaat9076. doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiely LJ, Busca K, Lane JA, van Sinderen D, Hickey RM. Molecular strategies for the utilisation of human milk oligosaccharides by infant gut-associated bacteria. FEMS Microbiol Rev. 2023;47(6). doi: 10.1093/femsre/fuad056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garrido D, Ruiz-Moyano S, Lemay DG, Sela DA, German JB, Mills DA. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci Rep. 2015;5(1):13517. doi: 10.1038/srep13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105(48):18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, Kumagai H, Ashida H, Hirose J, Kitaoka M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. 2011;286(40):34583–34592. doi: 10.1074/jbc.M111.248138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gotoh A, Katoh T, Sakanaka M, Ling Y, Yamada C, Asakuma S, Urashima T, Tomabechi Y, Katayama-Ikegami A, Kurihara S, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep. 2018;8(1):13958. doi: 10.1038/s41598-018-32080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermes GDA, Rasmussen C, Wellejus A. Variation in the conservation of species-specific gene sets for HMO degradation and its effects on HMO utilization in bifidobacteria. Nutrients. 2024;16(12):1893. doi: 10.3390/nu16121893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turroni F, Bottacini F, Foroni E, Mulder I, Kim J-H, Zomer A, Sánchez B, Bidossi A, Ferrarini A, Giubellini V, et al. Genome analysis of bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci USA. 2010;107(45):19514–19519. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, Mills DA. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl Environ Microbiol. 2013;79(19):6040–6049. doi: 10.1128/AEM.01843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Díaz R, Torres-Miranda A, Orellana G, Garrido D. Comparative genomic analysis of novel Bifidobacterium longum subsp. longum strains reveals functional divergence in the human gut microbiota. Microorganisms. 2021;9(9):1906. doi: 10.3390/microorganisms9091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garrido D, Ruiz-Moyano S, Kirmiz N, Davis JC, Totten SM, Lemay DG, Ugalde JA, German JB, Lebrilla CB, Mills DA. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci Rep. 2016;6(1):35045. doi: 10.1038/srep35045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holst AQ, Jois H, Laursen MF, Sommer MOA, Licht TR, Bahl MI. Human milk oligosaccharides induce acute yet reversible compositional changes in the gut microbiota of conventional mice linked to a reduction of butyrate levels. Microlife. 2022;3:uqac006. doi: 10.1093/femsml/uqac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kijner S, Cher A, Yassour M. The infant gut commensal Bacteroides dorei presents a generalized transcriptional response to various human milk oligosaccharides. Front Cell Infect Microbiol. 2022;12:854122. doi: 10.3389/fcimb.2022.854122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salli K, Hirvonen J, Siitonen J, Ahonen I, Anglenius H, Maukonen J. Selective utilization of the human milk oligosaccharides 2’-fucosyllactose, 3-fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria. J Agric Food Chem. 2021;69(1):170–182. doi: 10.1021/acs.jafc.0c06041. [DOI] [PubMed] [Google Scholar]
- 79.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao Y, Davis B, Zhu W, Zheng N, Meng D, Walker WA. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am J Physiol Gastrointest Liver Physiol. 2021;320(4):G521–G530. doi: 10.1152/ajpgi.00279.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci USA. 2020;117(21):11648–11657. doi: 10.1073/pnas.1917597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M, Lu H, Xue Y, Ning Y, Yuan Q, Li H, He Y, Jia X, Wang S. An in vitro colonic fermentation study of the effects of human milk oligosaccharides on gut microbiota and short-chain fatty acid production in infants aged 0–6 months. Foods. 2024;13(6):921. doi: 10.3390/foods13060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan H, Chen Y, Zhu H, Huang W-H, Cai X-H, Li D, Lv Y-J, Si-Zhao, Zhou H-H, Luo F-Y, et al. The relationship among intestinal bacteria, vitamin K and response of vitamin K antagonist: A review of evidence and potential mechanism. Front Med. 2022;9:829304. doi: 10.3389/fmed.2022.829304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellis JL, Wang M, Fu X, Fields CJ, Donovan SM, Booth SL. Feeding practice and delivery mode are determinants of vitamin K in the infant gut: An exploratory analysis. Curr Dev Nutr. 2022;6(3):nzac019. doi: 10.1093/cdn/nzac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, et al. Stereotypic immune system development in newborn children. Cell. 2018;174(5):1277–1292.e14. doi: 10.1016/j.cell.2018.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, Xu G, Davis JCC, Lebrilla CB, Henrick BM, et al. Persistence of Supplemented Bifidobacterium longum subsp. infantis EVC001 in Breastfed Infants. mSphere. 2017;2(6). doi: 10.1128/mSphere.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11(8):2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 91.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 92.Capeding MRZ, Phee LCM, Ming C, Noti M, Vidal K, Le Carrou G, Frézal A, Moll JM, Vogt JK, Myers PN, et al. Safety, efficacy, and impact on gut microbial ecology of a Bifidobacterium longum subspecies infantis LMG11588 supplementation in healthy term infants: a randomized, double-blind, controlled trial in the Philippines. Front Nutr. 2023;10:1319873. doi: 10.3389/fnut.2023.1319873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casaburi G, Duar RM, Vance DP, Mitchell R, Contreras L, Frese SA, Smilowitz JT, Underwood MA. Early-life gut microbiome modulation reduces the abundance of antibiotic-resistant bacteria. Antimicrob Resist Infect Control. 2019;8(1):131. doi: 10.1186/s13756-019-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Casaburi G, Frese SA. Colonization of breastfed infants by Bifidobacterium longum subsp. infantis EVC001 reduces virulence gene abundance. Hum Microbiome J. 2018;9:7–10. doi: 10.1016/j.humic.2018.05.001. [DOI] [Google Scholar]
- 95.Hiraku A, Nakata S, Murata M, Xu C, Mutoh N, Arai S, Odamaki T, Iwabuchi N, Tanaka M, Tsuno T, et al. Early probiotic supplementation of healthy term infants with Bifidobacterium longum subsp. infantis M-63 is safe and leads to the development of Bifidobacterium-predominant gut microbiota: A double-blind, placebo-controlled trial. Nutrients. 2023;15(6):1402. doi: 10.3390/nu15061402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, Chang Y, Florez ID, Foroutan F, Shahid S, Zeraatkar D, et al. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: A systematic review and network meta-analysis of randomized trials. Gastroenterology. 2020;159(2):467–480. doi: 10.1053/j.gastro.2020.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bachmann V, Kostiuk B, Unterweger D, Diaz-Satizabal L, Ogg S, Pukatzki S, Picardeau M. Bile salts modulate the mucin-activated type VI secretion system of pandemic Vibrio cholerae. PLOS Negl Trop Dis. 2015;9(8):e0004031. doi: 10.1371/journal.pntd.0004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gagnon M, Kheadr EE, Le Blay G, Fliss I. In vitro inhibition of Escherichia coli O157: H7 by bifidobacterial strains of human origin. Int J Food Microbiol. 2004;92(1):69–78. doi: 10.1016/j.ijfoodmicro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 99.Trejo FM, Minnaard J, Perez PF, De Antoni GL. Inhibition of Clostridium difficile growth and adhesion to enterocytes by Bifidobacterium supernatants. Anaerobe. 2006;12(4):186–193. doi: 10.1016/j.anaerobe.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184(15):3884–3898.e11. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 101.Méndez CS, Bueno SM, Kalergis AM, Xu B. Contribution of gut microbiota to immune tolerance in infants. J Immunol Res. 2021;2021:1–11. doi: 10.1155/2021/7823316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5(1):4. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korpela K, de Vos WM. Early life colonization of the human gut: microbes matter everywhere. Curr Opin Microbiol. 2018;44:70–78. doi: 10.1016/j.mib.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, Arp K, Watson RL, Sanders EAM, Fuentes S, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. doi: 10.1038/s41467-019-13014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adeyeye TE, Yeung EH, McLain AC, Lin S, Lawrence DA, Bell EM. Wheeze and food allergies in children born via cesarean delivery: The Upstate KIDS study. Am J Epidemiol. 2019;188(2):355–362. doi: 10.1093/aje/kwy257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos A-M, Kunøe A, Fink NR, Chawes BL, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Tapiainen T, Brinkac L, Lorenzi HA, Moncera K, Tejesvi MV, Salo J, Nelson KE. Vertical transmission of gut microbiome and antimicrobial resistance genes in infants exposed to antibiotics at birth. J Infect Dis. 2021;224(7):1236–1246. doi: 10.1093/infdis/jiaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazzola G, Murphy K, Ross RP, Di Gioia D, Biavati B, Corvaglia LT, Faldella G, Stanton C, Wilson BA. Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLOS ONE. 2016;11(6):e0157527. doi: 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, Fernández N, Alaez L, Hernández-Barranco AM, de Los Reyes-Gavilán CG, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. 2017;5(1):93. doi: 10.1186/s40168-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou Z-H, Liu D, Li H-D, Zhu D-P, He Y, Hou T, Yu J-L. Prenatal and postnatal antibiotic exposure influences the gut microbiota of preterm infants in neonatal intensive care units. Ann Clin Microbiol Antimicrob. 2018;17(1):9. doi: 10.1186/s12941-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tapiainen T, Koivusaari P, Brinkac L, Lorenzi HA, Salo J, Renko M, Pruikkonen H, Pokka T, Li W, Nelson K, et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci Rep. 2019;9(1):10635. doi: 10.1038/s41598-019-46964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beller L, Deboutte W, Falony G, Vieira-Silva S, Tito RY, Valles-Colomer M, Rymenans L, Jansen D, Van Espen L, Papadaki MI, et al. Successional stages in infant gut microbiota maturation. MBio. 2021;12(6):e0185721. doi: 10.1128/mBio.01857-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yassour M, Vatanen T, Siljander H, Hämäläinen A-M, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duong QA, Pittet LF, Curtis N, Zimmermann P. Antibiotic exposure and adverse long-term health outcomes in children: A systematic review and meta-analysis. J Infect. 2022;85(3):213–300. doi: 10.1016/j.jinf.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Kim DH, Han K, Kim SW. Effects of antibiotics on the development of asthma and other allergic diseases in children and adolescents. Allergy Asthma Immunol Res. 2018;10(5):457–465. doi: 10.4168/aair.2018.10.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, Bender JM, Azad MB, Thompson AL, Weiss ST, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):4169. doi: 10.1038/s41467-018-06473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jian C, Carpén N, Helve O, de Vos WM, Korpela K, Salonen A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. EBioMedicine. 2021;69:103475. doi: 10.1016/j.ebiom.2021.103475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lv H, Zhang L, Han Y, Wu L, Wang B. The development of early life microbiota in human health and disease. Proc Est Acad Sci Eng. 2022;12:101–114. doi: 10.1016/j.eng.2020.12.014. [DOI] [Google Scholar]
- 119.Dai DLY, Petersen C, Hoskinson C, Del Bel KL, Becker AB, Moraes TJ, Mandhane PJ, Finlay BB, Simons E, Kozyrskyj AL, et al. Breastfeeding enrichment of B. longum subsp. infantis mitigates the effect of antibiotics on the microbiota and childhood asthma risk. Med (NY). 2023;4(2):92–112.e5. doi: 10.1016/j.medj.2022.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Carr LE, Virmani MD, Rosa F, Munblit D, Matazel KS, Elolimy AA, Yeruva L. Role of human milk bioactives on infants’ gut and immune health. Front Immunol. 2021;12:604080. doi: 10.3389/fimmu.2021.604080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13(1):12–21. doi: 10.1038/s41385-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pärnänen KMM, Hultman J, Markkanen M, Satokari R, Rautava S, Lamendella R, Wright J, McLimans CJ, Kelleher SL, Virta MP. Early-life formula feeding is associated with infant gut microbiota alterations and an increased antibiotic resistance load. Am J Clin Nutr. 2022;115(2):407–421. doi: 10.1093/ajcn/nqab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Sullivan A, Farver M, Smilowitz JT. Correction to “The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants”. Nutr Metab Insights. 2015;8(Suppl 1):1–9. doi: 10.4137/NMI.S41125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parkin K, Palmer DJ, Verhasselt V, Amenyogbe N, Cooper MN, Christophersen CT, Prescott SL, Silva D, Martino D. Metagenomic characterisation of the gut microbiome and effect of complementary feeding on Bifidobacterium spp Australian Infants. Microorganisms. 2024;12(1):228. doi: 10.3390/microorganisms12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Differding MK, Benjamin-Neelon SE, Hoyo C, T Ø, Mueller NT. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020;20(1):56. doi: 10.1186/s12866-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quin C, Vicaretti SD, Mohtarudin NA, Garner AM, Vollman DM, Gibson DL, Zandberg WF. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J Biol Chem. 2020;295(12):4035–4048. doi: 10.1074/jbc.RA119.011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beckers KF, Flanagan JP, Sones JL. Microbiome and pregnancy: focus on microbial dysbiosis coupled with maternal obesity. Int J Obes. 2024;48(4):439–448. doi: 10.1038/s41366-023-01438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubini E, Schenkelaars N, Rousian M, Sinclair KD, Wekema L, Faas MM, Steegers-Theunissen RPM, Schoenmakers S. Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: Implications for fetal development and offspring wellbeing. Am J Obstet Gynecol. 2022;227(3):392–400. doi: 10.1016/j.ajog.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 129.Singh P, Elhaj DAI, Ibrahim I, Abdullahi H, Al Khodor S. Maternal microbiota and gestational diabetes: impact on infant health. J Transl Med. 2023;21(1):364. doi: 10.1186/s12967-023-04230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, Ji P, Zhang F, Jia Z, Wang Y, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67(9):1614–1625. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilley SP, Ruebel ML, Sims C, Zhong Y, Turner D, Lan RS, Pack LM, Piccolo BD, Chintapalli SV, Abraham A, et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr Obes. 2022;17(9):e12921. doi: 10.1111/ijpo.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stanislawski MA, Dabelea D, Wagner BD, Iszatt N, Dahl C, Sontag MK, Knight R, Lozupone CA, Eggesbø M. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a Norwegian birth cohort. MBio. 2018;9(5). doi: 10.1128/mBio.01751-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang M, Marroquin E. The role of the gut microbiome in the intergenerational transmission of the obesity phenotype: A narrative review. Front Med. 2022;9:1057424. doi: 10.3389/fmed.2022.1057424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Cuna A, Morowitz MJ, Ahmed I, Umar S, Sampath V. Dynamics of the preterm gut microbiome in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;320(4):G411–G419. doi: 10.1152/ajpgi.00399.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cifuentes MP, Chapman JA, Stewart CJ. Gut microbiome derived short chain fatty acids: Promising strategies in necrotising enterocolitis. Curr Res Microb Sci. 2024;6:100219. doi: 10.1016/j.crmicr.2024.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gueimonde M, Sakata S, Kalliomäki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of Lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42(2):166–170. doi: 10.1002/j.1536-4801.2006.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 138.Tang MLK, Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins-Browne RM, Salminen S. Prenatal administration of Lactobacillus rhamnosus GG can modulate intestinal microbiota in infants at high risk of allergic disease. J Allergy Clin Immunol. 2009;123(2):S224–S224. doi: 10.1016/j.jaci.2008.12.859. [DOI] [PubMed] [Google Scholar]
- 139.O’Brien CE, Meier AK, Cernioglo K, Mitchell RD, Casaburi G, Frese SA, Henrick BM, Underwood MA, Smilowitz JT. Early probiotic supplementation with B. infantis in breastfed infants leads to persistent colonization at 1 year. Pediatr Res. 2022;91(3):627–636. doi: 10.1038/s41390-020-01350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gong Y, Zhong H, Wang J, Wang X, Huang L, Zou Y, Qin H, Yang R. Effect of probiotic supplementation on the gut microbiota composition of infants delivered by cesarean section: An exploratory, randomized, open-label, parallel-controlled trial. Curr Microbiol. 2023;80(11):341. doi: 10.1007/s00284-023-03444-4. [DOI] [PubMed] [Google Scholar]
- 141.Korpela K, Salonen A, Vepsäläinen O, Suomalainen M, Kolmeder C, Varjosalo M, Miettinen S, Kukkonen K, Savilahti E, Kuitunen M, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6(1):182. doi: 10.1186/s40168-018-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beck LC, Masi AC, Young GR, Vatanen T, Lamb CA, Smith R, Coxhead J, Butler A, Marsland BJ, Embleton ND, et al. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat Microbiol. 2022;7(10):1525–1535. doi: 10.1038/s41564-022-01213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Samara J, Moossavi S, Alshaikh B, Ortega VA, Pettersen VK, Ferdous T, Hoops SL, Soraisham A, Vayalumkal J, Dersch-Mills D, et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host & Microbe. 2022;30(5):696–711.e5. doi: 10.1016/j.chom.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 144.Alliet P, Vandenplas Y, Roggero P, Jespers SNJ, Peeters S, Stalens J-P, Kortman GAM, Amico M, Berger B, Sprenger N, et al. Safety and efficacy of a probiotic-containing infant formula supplemented with 2’-fucosyllactose: a double-blind randomized controlled trial. Nutr J. 2022;21(1):11. doi: 10.1186/s12937-022-00764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berger B, Porta N, Foata F, Grathwohl D, Delley M, Moine D, Charpagne A, Siegwald L, Descombes P, Alliet P, et al. Linking human milk oligosaccharides, infant fecal community types, and later risk to require antibiotics. MBio. 2020;11(2). doi: 10.1128/mBio.03196-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bosheva M, Tokodi I, Krasnow A, Pedersen HK, Lukjancenko O, Eklund AC, Grathwohl D, Sprenger N, Berger B, Cercamondi CI, et al. Infant formula with a specific blend of five human milk oligosaccharides drives the gut microbiota development and improves gut maturation markers: A randomized controlled trial. Front Nutr. 2022;9:920362. doi: 10.3389/fnut.2022.920362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garcia Rodenas CL, Lepage M, Ngom-Bru C, Fotiou A, Papagaroufalis K, Berger B. Effect of formula containing Lactobacillus reuteri DSM 17938 on fecal microbiota of infants born by cesarean-section. J Pediatr Gastroenterol Nutr. 2016;63(6):681–687. doi: 10.1097/MPG.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 148.Lagkouvardos I, Intze E, Schaubeck M, Rooney JP, Hecht C, Piloquet H, Clavel T. Early life gut microbiota profiles linked to synbiotic formula effects: a randomized clinical trial in European infants. Am J Clin Nutr. 2023;117(2):326–339. doi: 10.1016/j.ajcnut.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 149.Lay C, Chu CW, Purbojati RW, Acerbi E, Drautz-Moses DI, de Sessions PF, Jie S, Ho E, Kok YJ, Bi X, et al. A synbiotic intervention modulates meta-omics signatures of gut redox potential and acidity in elective caesarean born infants. BMC Microbiol. 2021;21(1):191. doi: 10.1186/s12866-021-02230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. 2018;9(3):189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Halkjær SI, Refslund Danielsen M, de Knegt VE, Andersen LO, Stensvold CR, Nielsen HV, Mirsepasi-Lauridsen HC, Krogfelt KA, Cortes D, Petersen AM. Multi-strain probiotics during pregnancy in women with obesity influence infant gut microbiome development: Results from a randomized, double-blind placebo-controlled study. Gut Microbes. 2024;16(1):2337968. doi: 10.1080/19490976.2024.2337968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(1):192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 153.Niers L, Martín R, Rijkers G, Sengers F, Timmerman H, van Uden N, Smidt H, Kimpen J, Hoekstra M. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy. 2009;64(9):1349–1358. doi: 10.1111/j.1398-9995.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 154.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021;10(3):459. doi: 10.3390/jcm10030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Falcon RMG, Caoili SEC. Immunologic, genetic, and ecological interplay of factors involved in allergic diseases. Front Allergy. 2023;4:1215616. doi: 10.3389/falgy.2023.1215616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, Rossi AB, Brignoli L, Saba G, Guillemin I, et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–428.e2. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 157.Miltner LA, Vonk JM, van der Velde JL, Sprikkelman AB. Eczema in early childhood increases the risk of allergic multimorbidity. Clin Transl Allergy. 2024;14(9):e12384. doi: 10.1002/clt2.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Galazzo G, van Best N, Bervoets L, Dapaah IO, Savelkoul PH, Hornef MW, GI-MDH consortium, Lau S, Hamelmann E, Penders J, et al. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology. 2020;158(6):1584–1596. doi: 10.1053/j.gastro.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 159.Park YM, Lee SY, Kang MJ, Kim BS, Lee MJ, Jung SS, Yoon JS, Cho HJ, Lee E, Yang SI, et al. Imbalance of gut Streptococcus, Clostridium, and Akkermansia determines the natural course of atopic dermatitis in infant. Allergy Asthma Immunol Res. 2020;12(2):322–337. doi: 10.4168/aair.2020.12.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov. sp. nov. a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 161.Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: When, where, and how? Microorganisms. 2018;6(3):75. doi: 10.3390/microorganisms6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 163.Zhou Q, Pang G, Zhang Z, Yuan H, Chen C, Zhang N, Yang Z, Sun L. Association between gut Akkermansia and metabolic syndrome is dose-dependent and affected by microbial interactions: A cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:2177–2188. doi: 10.2147/DMSO.S311388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Q, Zhang Y, Wang X, Yang R, Zhu X, Zhang Y, Chen C, Yuan H, Yang Z, Sun L. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project. Nutr Metab (Lond). 2020;17(1):90. doi: 10.1186/s12986-020-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Si J, Kang H, You HJ, Ko G. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes. 2022;14(1):2078619. doi: 10.1080/19490976.2022.2078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ta LDH, Chan JCY, Yap GC, Purbojati RW, Drautz-Moses DI, Koh YM, Tay CJX, Huang C-H, Kioh DYQ, Woon JY, et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes. 2020;12(1):1–22. doi: 10.1080/19490976.2020.1801964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leo S, Cetiner OF, Pittet LF, Messina NL, Jakob W, Falquet L, Curtis N, Zimmermann P. The association between the composition of the early-life intestinal microbiome and eczema in the first year of life. Front Microbiomes. 2024;2. doi: 10.3389/frmbi.2023.1147082. [DOI] [Google Scholar]
- 168.Kang MJ, Lee SY, Park YM, Kim BS, Lee MJ, Kim JH, Jeong S, Lee SH, Park MJ, Rhee ES, et al. Interactions between IL-17 variants and Streptococcus in the gut contribute to the development of atopic dermatitis in infancy. Allergy Asthma Immunol Res. 2021;13(3):404–419. doi: 10.4168/aair.2021.13.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Korpela K, Hurley S, Ford SA, Franklin R, Byrne S, Lunjani N, Forde B, Neogi U, Venter C, Walter J, et al. Association between gut microbiota development and allergy in infants born during pandemic-related social distancing restrictions. Allergy. 2024;79(7):1938–1951. doi: 10.1111/all.16069. [DOI] [PubMed] [Google Scholar]
- 170.Cheng HY, Chan JCY, Yap GC, Huang C-H, Kioh DYQ, Tham EH, Loo EXL, Shek LPC, Karnani N, Goh A, et al. Evaluation of stool short chain fatty acids profiles in the first year of life with childhood atopy-related outcomes. Front Allergy. 2022;3:873168. doi: 10.3389/falgy.2022.873168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zablotsky B, Black L, Akinbami L. Diagnosed allergic conditions in children aged 0–17 years: United States, 2021. Centers for Disease Control and Prevention; 2023. doi: 10.15620/cdc:123250. [DOI] [PubMed] [Google Scholar]
- 172.Ng A, Boersma P. Diagnosed allergic conditions in adults: United States, 2021. National Center for Health Statistics (U.S.); 2023. doi: 10.15620/cdc:122809. [DOI] [PubMed] [Google Scholar]
- 173.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, Wang L, Li N, Crestani E, Spielman S, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med. 2019;25(7):1164–1174. doi: 10.1038/s41591-019-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25(3):448–453. doi: 10.1038/s41591-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S, et al. Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol. 2016;138(4):1122–1130. doi: 10.1016/j.jaci.2016.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hirsch AG, Pollak J, Glass TA, Poulsen MN, Bailey-Davis L, Mowery J, Schwartz BS. Early-life antibiotic use and subsequent diagnosis of food allergy and allergic diseases. Clin Exp Allergy. 2017;47(2):236–244. doi: 10.1111/cea.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Papathoma E, Triga M, Fouzas S, Dimitriou G. Cesarean section delivery and development of food allergy and atopic dermatitis in early childhood. Pediatr Allergy Immunol. 2016;27(4):419–424. doi: 10.1111/pai.12552. [DOI] [PubMed] [Google Scholar]
- 179.Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, Sandel M, Bacharier LB, Zeiger R, Sodergren E, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy. 2018;73(1):145–152. doi: 10.1111/all.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wampach L, Heintz-Buschart A, Fritz JV, Ramiro-Garcia J, Habier J, Herold M, Narayanasamy S, Kaysen A, Hogan AH, Bindl L, et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9(1):5091. doi: 10.1038/s41467-018-07631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goldberg MR, Mor H, Magid Neriya D, Magzal F, Muller E, Appel MY, Nachshon L, Borenstein E, Tamir S, Louzoun Y, et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020;12(1):92. doi: 10.1186/s13073-020-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance - United States, 2006–2018. MMWR Surveill Summ. 2021;70(5):1–32. doi: 10.15585/mmwr.ss7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Durack J, Kimes NE, Lin DL, Rauch M, McKean M, McCauley K, Panzer AR, Mar JS, Cabana MD, Lynch SV. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun. 2018;9(1):707. doi: 10.1038/s41467-018-03157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stokholm J, Thorsen J, Blaser MJ, Rasmussen MA, Hjelmsø M, Shah S, Christensen ED, Chawes BL, Bønnelykke K, Brix S, et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci Transl Med. 2020;12(569):eaax9929. doi: 10.1126/scitranslmed.aax9929. [DOI] [PubMed] [Google Scholar]
- 185.Arrieta M-C, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, Vaca M, Boutin RCT, Morien E, Jin M, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2017. Dec. 142(2):424–434.e10. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, Aguilera M, Khanna S, Gil A, Edwards CA, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51(1):77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 187.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 188.Davis-Richardson AG, Ardissone AN, Dias R, Simell V, Leonard MT, Kemppainen KM, Drew JC, Schatz D, Atkinson MA, Kolaczkowski B, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol. 2014;5:678. doi: 10.3389/fmicb.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, Castellano-Castillo D, Moreno-Indias I, Urda-Cardona A, Tinahones FJ, Fernández-García JC, Queipo-Ortuño MI. Gut microbiota differs in composition and functionality between children with type 1 Diabetes and MODY2 and healthy control subjects: A case-control study. Diabetes Care. 2018;41(11):2385–2395. doi: 10.2337/dc18-0253. [DOI] [PubMed] [Google Scholar]
- 190.Zreloff ZJ, Lange D, Vernon SD, Carlin MR, Cano RJ. Accelerating gut microbiome research with robust sample collection. Res Rev J Microbiol Biotechnol. 2023;12(1):33–47. https://pubmed.ncbi.nlm.nih.gov/3738857. [PMC free article] [PubMed] [Google Scholar]
- 191.Panek M, Čipčić Paljetak H, Barešić A, Perić M, Matijašić M, Lojkić I, Vranešić Bender D, Krznarić Ž, Verbanac D. Methodology challenges in studying human gut microbiota - effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci Rep. 2018;8(1):5143. doi: 10.1038/s41598-018-23296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. 2017;8(1):1784. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Abdill RJ, Adamowicz EM, Blekhman R, Cadwell K. Public human microbiome data are dominated by highly developed countries. PLOS Biol. 2022;20(2):e3001536. doi: 10.1371/journal.pbio.3001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Flores GE, Caporaso JG, Henley JB, Rideout JR, Domogala D, Chase J, Leff JW, Vázquez-Baeza Y, Gonzalez A, Knight R, et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 2014;15(12):531. doi: 10.1186/s13059-014-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uhr GT, Dohnalová L, Thaiss CA. The dimension of time in host-microbiome interactions. mSystems. 2019;4(1). doi: 10.1128/mSystems.00216-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19(5):731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 199.Tavalire HF, Christie DM, Leve LD, Ting N, Cresko WA, Bohannan BJM, Fraser CM. Shared environment and genetics shape the gut microbiome after infant adoption. MBio. 2021;12(2). doi: 10.1128/mBio.00548-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, Ward KJ, Jackson MA, Xia Y, Chen X, et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 2016;3(6):572–584.e3. doi: 10.1016/j.cels.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gacesa R, Kurilshikov A, Vich Vila A, Sinha T, Klaassen MAY, Bolte LA, Andreu-Sánchez S, Chen L, Collij V, Hu S, et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022;604(7907):732–739. doi: 10.1038/s41586-022-04567-7. [DOI] [PubMed] [Google Scholar]
- 202.Tian L, Wang X-W, Wu A-K, Fan Y, Friedman J, Dahlin A, Waldor MK, Weinstock GM, Weiss ST, Liu Y-Y. Deciphering functional redundancy in the human microbiome. Nat Commun. 2020;11(1):6217. doi: 10.1038/s41467-020-19940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim N, Ma J, Kim W, Kim J, Belenky P, Lee I. Genome-resolved metagenomics: a game changer for microbiome medicine. Exp Mol Med. 2024;56(7):1501–1512. doi: 10.1038/s12276-024-01262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alcazar C-M, Paes VM, Shao Y, Oesser C, Miltz A, Lawley TD, Brocklehurst P, Rodger A, Field N. The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe. 2022;3(11):e867–e880. doi: 10.1016/S2666-5247(22)00184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study


